Diffuse Cutaneous Systemic Sclerosis Market Forecast: Opportunities and Growth Trends Upto 2034 | DelveInsight

The diffuse cutaneous systemic sclerosis market landscape is evolving from conventional symptom-focused strategies toward therapies that specifically target fibrosis and immune modulation, addressing a significant unmet need. The development pipeline is varied, with prominent candidates including Mitsubishi Tanabe Pharma’s MT-7117, Cumberland’s ifetroban, Johnson & Johnson’s guselkumab, and Kyverna’s KYV-101. The dcSSc market is anticipated to move toward earlier intervention with targeted therapies, combination approaches, and precision medicine.
New York, USA, Oct. 06, 2025 (GLOBE NEWSWIRE) — Diffuse Cutaneous Systemic Sclerosis Market Forecast: Opportunities and Growth Trends Upto 2034 | DelveInsight
The diffuse cutaneous systemic sclerosis market landscape is evolving from conventional symptom-focused strategies toward therapies that specifically target fibrosis and immune modulation, addressing a significant unmet need. The development pipeline is varied, with prominent candidates including Mitsubishi Tanabe Pharma’s MT-7117, Cumberland’s ifetroban, Johnson & Johnson’s guselkumab, and Kyverna’s KYV-101. The dcSSc market is anticipated to move toward earlier intervention with targeted therapies, combination approaches, and precision medicine.
DelveInsight’s Diffuse Cutaneous Systemic Sclerosis Market Insights report includes a comprehensive understanding of current treatment practices, emerging diffuse cutaneous systemic sclerosis drugs, market share of individual therapies, and current and forecasted diffuse cutaneous systemic sclerosis market size from 2020 to 2034, segmented into leading markets (the US, EU4, UK, and Japan).
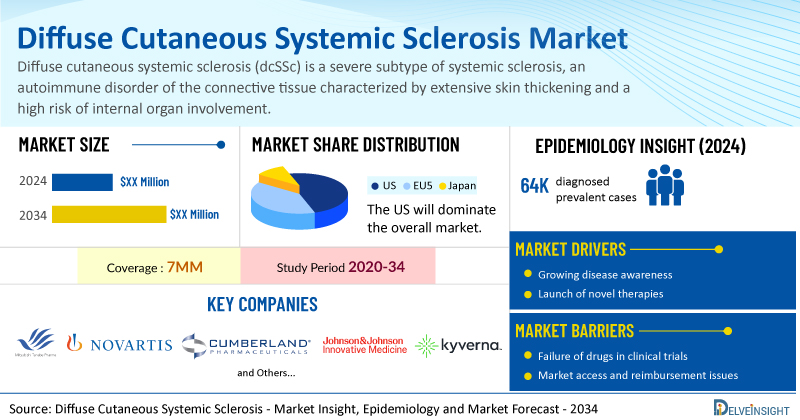
Diffuse Cutaneous Systemic Sclerosis Market Summary
- The total diffuse cutaneous systemic sclerosis treatment market size is expected to grow positively by 2034 in the leading markets.
- The United States accounts for the largest market size of diffuse cutaneous systemic sclerosis, in comparison to EU4 (Germany, Italy, France, and Spain), the UK, and Japan.
- The total number of diagnosed prevalent cases of dcSSc in the leading markets were about ~64K in 2024.
- Key diffuse cutaneous systemic sclerosis companies, including Mitsubishi Tanabe Pharma, Novartis, Cumberland Pharmaceuticals, Johnson & Johnson Innovative Medicine, Kyverna Therapeutics, and others, are actively working on innovative diffuse cutaneous systemic sclerosis drugs.
- Some of the key diffuse cutaneous systemic sclerosis therapies in clinical trials include Dersimelagon (MT-7117), Ianalumab, Vasculan (ifetroban), Guselkumab, KYV 101, and others. These novel diffuse cutaneous systemic sclerosis therapies are anticipated to enter the diffuse cutaneous systemic sclerosis market in the forecast period and are expected to change the market.
Discover which diffuse cutaneous systemic sclerosis medications are expected to grab the market share @ Diffuse Cutaneous Systemic Sclerosis Market Report
Key Factors Driving the Growth of the Diffuse Cutaneous Systemic Sclerosis Market
Rising Global Prevalence and Awareness
Systemic sclerosis, including its diffuse cutaneous form, is a rare autoimmune disease characterized by excessive collagen deposition, leading to skin thickening and potential organ fibrosis. The prevalence of dcSSc is estimated at approximately 1 in 25,000 adults, with a higher incidence in women (female-to-male ratio of around 4:1). This growing recognition is prompting increased research and development efforts in the field.
Improved Diagnostic Techniques
Advancements in diagnostic technologies are enabling earlier and more accurate detection of dcSSc. Early diagnosis is critical for initiating timely interventions, which can significantly improve patient outcomes. Enhanced diagnostic capabilities are also contributing to a better understanding of the disease’s epidemiology and progression.
Advancement in dcSSc Drug Development
Some of the promising companies, such as Mitsubishi Tanabe Pharma (MT-7117), Cumberland Pharmaceuticals (ifetroban), Johnson & Johnson (Guselkumab), Kyverna Therapeutics (KYV-101), and others, are evaluating their lead assets in different stages of development.
Diffuse Cutaneous Systemic Sclerosis Market Analysis
Currently, there are no therapies that can cure or fundamentally alter the course of the disease. Long-term treatments often lose effectiveness over time and are associated with significant adverse effects. As a result, current management strategies primarily focus on alleviating symptoms in affected organs, with approaches tailored to disease severity, progression, and duration.
Biologic therapies are emerging as promising alternatives, either as standalone or adjunct treatments, particularly when combined with agents that have anti-fibrotic properties. The dcSSc treatment landscape is gradually shifting from traditional symptom-based management toward targeted antifibrotic and immunomodulatory strategies to address substantial unmet needs.
Approved therapies include OFEV (nintedanib, Boehringer Ingelheim), the first antifibrotic shown to slow lung function decline in SSc-ILD; ACTEMRA (tocilizumab, Roche), which modulates IL-6–driven inflammation and lung outcomes; and RITUXAN (rituximab, Zenyaku Kogyo/Chugai), a B-cell–depleting therapy increasingly used in clinical practice.
Investigational therapies in dcSSc target various pathogenic pathways using distinct mechanisms. MC1R agonists regulate inflammation, vascular dysfunction, and fibrosis via the melanocortin 1 receptor. BAFF inhibitors reduce the survival of abnormal B cells and autoantibody production. TxA2/PGH2 antagonists inhibit thromboxane and prostaglandin signaling, thereby reducing vasoconstriction, platelet aggregation, and fibrosis.
IL-23 inhibitors modulate the IL-23/Th17 axis to suppress pro-inflammatory responses associated with disease progression. CD19-directed therapies deplete pathogenic B cells, thereby restoring immune balance and limiting autoimmune activity. Collectively, these novel approaches aim to go beyond conventional immunosuppressants by targeting the key drivers of dcSSc pathogenesis.
Learn more about the diffuse cutaneous systemic sclerosis treatment options @ Diffuse Cutaneous Systemic Sclerosis Treatment Market
Diffuse Cutaneous Systemic Sclerosis Competitive Landscape
The treatment pipeline for dcSSc reflects a significant unmet medical need. A prominent key player, Mitsubishi Tanabe Pharma (MT-7117), Cumberland Pharmaceuticals (ifetroban), Johnson & Johnson (Guselkumab), Kyverna Therapeutics (KYV-101), and others are currently active in the dcSSc treatment space.
Mitsubishi Tanabe Pharma’s Dersimelagon is an orally administered, synthetic, non-peptide small molecule under development for multiple therapeutic indications. It functions as a selective MC1R agonist, exerting anti-inflammatory, anti-fibrotic, and vascular-protective effects by acting on inflammatory cells, endothelial cells, and fibroblasts. This profile positions it as a potential treatment option for systemic sclerosis. In April 2020, Mitsubishi Tanabe Pharma received Fast Track Designation for MT-7117 for the treatment of diffuse cutaneous systemic sclerosis.
Novartis’ Ianalumab is a novel monoclonal antibody that targets the B-cell activating factor receptor (BAFF-R). It aims to reduce B-cell populations through antibody-dependent cellular cytotoxicity (ADCC) and by blocking survival signals for B cells. Since B cells play a key role in the pathogenesis of diffuse cutaneous systemic sclerosis, including autoantibody production and profibrotic signaling, Ianalumab represents a targeted immunomodulatory strategy.
The anticipated launch of these emerging diffuse cutaneous systemic sclerosis therapies are poised to transform the diffuse cutaneous systemic sclerosis market landscape in the coming years. As these cutting-edge diffuse cutaneous systemic sclerosis therapies continue to mature and gain regulatory approval, they are expected to reshape the diffuse cutaneous systemic sclerosis market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for diffuse cutaneous systemic sclerosis, visit @ Diffuse Cutaneous Systemic Sclerosis Medication
Recent Developments in the Diffuse Cutaneous Systemic Sclerosis Market
- In July 2025, Novartis began a Phase II multicenter clinical study to assess the effectiveness, safety, and tolerability of Ianalumab in individuals with dcSSc.
- In May 2025, Mitsubishi Tanabe Pharma America announced the completion of enrollment for its Phase III INSPIRE Study of Investigational Dersimelagon in Patients with EPP and XLP.
What is Diffuse Cutaneous Systemic Sclerosis?
Diffuse cutaneous systemic sclerosis (dcSSc) is a severe subtype of systemic sclerosis, an autoimmune disorder of the connective tissue characterized by extensive skin thickening and a high risk of internal organ involvement. The condition results from immune system activation, vascular damage, and abnormal collagen accumulation, causing fibrosis in both the skin and internal organs. Skin changes advance quickly, affecting the face, trunk, and limbs, often beginning with Raynaud’s phenomenon. Internal organ complications can include interstitial lung disease, pulmonary arterial hypertension, scleroderma renal crisis, heart dysfunction, and gastrointestinal motility issues.
Diffuse Cutaneous Systemic Sclerosis Epidemiology Segmentation
The diffuse cutaneous systemic sclerosis epidemiology section provides insights into the historical and current diffuse cutaneous systemic sclerosis patient pool and forecasted trends for the leading markets. Patients with dcSSc tend to have a more aggressive clinical course and eventually develop visceral complications (usually within the first 3 to 5 years), which, if severe, can lead to death.
The diffuse cutaneous systemic sclerosis market report proffers epidemiological analysis for the study period 2020–2034 in the leading markets segmented into:
- Total Diagnosed Prevalent Cases of Systemic Sclerosis
- Systemic Sclerosis by Disease Subset
- Age-specific Cases of Systemic Sclerosis
- Systemic Sclerosis With Organ Involvement
- Systemic Sclerosis Severity by Organ Damage
- Systemic Sclerosis Severity by Skin Thickness
Download the report to understand diffuse cutaneous systemic sclerosis management @ Diffuse Cutaneous Systemic Sclerosis Treatment Options
| Diffuse Cutaneous Systemic Sclerosis Market Report Metrics | Details |
| Study Period | 2020–2034 |
| Diffuse Cutaneous Systemic Sclerosis Market Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Diffuse Cutaneous Systemic Sclerosis Epidemiology Segmentation | Total Diagnosed Prevalent Cases of Systemic Sclerosis, Systemic Sclerosis by Disease Subset, Age-specific Cases of Systemic Sclerosis, Systemic Sclerosis With Organ Involvement, Systemic Sclerosis Severity by Organ Damage, and Systemic Sclerosis Severity by Skin Thickness |
| Key Diffuse Cutaneous Systemic Sclerosis Companies | Mitsubishi Tanabe Pharma, Novartis, Cumberland Pharmaceuticals, Johnson & Johnson Innovative Medicine, Kyverna Therapeutics, Boehringer Ingelheim, Roche, and others |
| Key Diffuse Cutaneous Systemic Sclerosis Therapies | Dersimelagon (MT-7117), Ianalumab, Vasculan (ifetroban), Guselkumab, KYV 101, OFEV, ACTEMRA, and others |
Scope of the Diffuse Cutaneous Systemic Sclerosis Market Report
- Diffuse Cutaneous Systemic Sclerosis Therapeutic Assessment: Diffuse Cutaneous Systemic Sclerosis current marketed and emerging therapies
- Diffuse Cutaneous Systemic Sclerosis Market Dynamics: Conjoint Analysis of Emerging Diffuse Cutaneous Systemic Sclerosis Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Diffuse Cutaneous Systemic Sclerosis Market Unmet Needs, KOL’s views, Analyst’s views, Diffuse Cutaneous Systemic Sclerosis Market Access and Reimbursement
Discover more about diffuse cutaneous systemic sclerosis drugs in development @ Diffuse Cutaneous Systemic Sclerosis Clinical Trials
Table of Contents
| 1 | Diffuse Cutaneous Systemic Sclerosis Market Key Insights |
| 2 | Diffuse Cutaneous Systemic Sclerosis Market Report Introduction |
| 3 | Epidemiology and Market Forecast Methodology |
| 4 | Diffuse Cutaneous Systemic Sclerosis (dcSSc): Market Overview at a Glance |
| 4.1 | Market Size (%) of Diffuse Cutaneous Systemic Sclerosis by therapies in 2024 |
| 4.2 | Market Size (%) of Diffuse Cutaneous Systemic Sclerosis by therapies in 2034 |
| 5 | Executive Summary |
| 6 | Key Events |
| 7 | Disease Background and Overview: Diffuse Cutaneous Systemic Sclerosis (dcSSc) |
| 7.1 | Introduction |
| 7.2 | Diffuse Cutaneous Systemic Sclerosis Causes |
| 7.3 | Diffuse Cutaneous Systemic Sclerosis Pathophysiology |
| 7.4 | Diffuse Cutaneous Systemic Sclerosis Symptoms |
| 7.5 | Diffuse Cutaneous Systemic Sclerosis Risk Factor |
| 7.6 | Diffuse Cutaneous Systemic Sclerosis Diagnosis |
| 8 | Diffuse Cutaneous Systemic Sclerosis Treatment and Management |
| 9 | Epidemiology and Patient Population |
| 9.1 | Key Findings |
| 9.2 | Assumptions and Rationale |
| 9.3 | Total Diagnosed Prevalent cases of dcSSc:7MM |
| 9.4 | The United States |
| 9.4.1 | Total Diagnosed Prevalent Cases of Systemic Sclerosis in the United States |
| 9.4.2 | Systemic Sclerosis by Disease Subset in the United States |
| 9.4.3 | Age-specific Cases of Systemic Sclerosis in the United States |
| 9.4.5 | Systemic Sclerosis With Organ Involvement in the United States |
| 9.4.6 | Systemic Sclerosis Severity by Organ Damage in the United States |
| 9.4.7 | Systemic Sclerosis Severity by Skin Thickness in the United States |
| 9.5 | EU4 and the UK |
| 9.6 | Japan |
| 10 | Patient Journey of Diffuse Cutaneous Systemic Sclerosis (dcSSc) |
| 11 | Marketed Diffuse Cutaneous Systemic Sclerosis Therapies |
| 11.1 | Key Competitors |
| 11.2 | OFEV (nintedanib): Boehringer Ingelheim |
| 11.2.1 | Product Description |
| 11.2.2 | Regulatory Milestones |
| 11.2.3 | Other Developmental Activities |
| 11.2.4 | Clinical Development |
| 11.2.4.1 | Clinical Trial Information |
| 11.2.5 | Safety and Efficacy |
| 11.2.6 | Analyst Views |
| 11.3 | ACTEMRA (tocilizumab): Roche |
| 12 | Emerging Diffuse Cutaneous Systemic Sclerosis Therapies |
| 12.1 | Key Cross Competition |
| 12.2 | Dersimelagon (MT-7117): Mitsubishi Tanabe Pharma |
| 12.2.1 | Product Description |
| 12.2.2 | Other Development Activities |
| 12.2.3 | Clinical Development Activities |
| 12.2.4 | Safety and Efficacy |
| 12.2.5 | Analyst View |
| 12.3 | Ianalumab: Novartis |
| 13 | Diffuse Cutaneous Systemic Sclerosis (dcSSc): Market Analysis |
| 13.1 | Key Findings |
| 13.2 | Diffuse Cutaneous Systemic Sclerosis Market Outlook |
| 13.3 | Conjoint Analysis |
| 13.4 | Key Market Forecast Assumptions |
| 13.5 | Total dcSSc Market Analysis: 7MM |
| 13.6 | United States Diffuse Cutaneous Systemic Sclerosis Market Size |
| 13.6.1 | Total Market Size of dcSSc in the United States |
| 13.6.2 | Market Size of dcSSc by Therapies in the United States |
| 13.7 | EU4 and the UK Diffuse Cutaneous Systemic Sclerosis Market Size |
| 13.8 | Japan Diffuse Cutaneous Systemic Sclerosis Market Size |
| 14 | Unmet Needs of Diffuse Cutaneous Systemic Sclerosis (dcSSc) |
| 15 | SWOT Analysis of Diffuse Cutaneous Systemic Sclerosis (dcSSc) |
| 16 | KOL Views of Diffuse Cutaneous Systemic Sclerosis (dcSSc) |
| 17 | Diffuse Cutaneous Systemic Sclerosis Market Access and Reimbursement |
| 17.1 | United States |
| 17.2 | EU4 and the UK |
| 17.3 | Japan |
| 18 | Bibliography |
| 19 | Diffuse Cutaneous Systemic Sclerosis Market Report Methodology |
Related Reports
Diffuse Cutaneous Systemic Sclerosis Clinical Trial Analysis
Diffuse Cutaneous Systemic Sclerosis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key dcSSc companies, including Kadmon Pharmaceuticals, Talaris Therapeutics, Horizon Therapeutics, Mitsubishi Tanabe Pharma, Takeda Oncology, Seagen, among others.
Systemic Sclerosis Market
Systemic Sclerosis Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key systemic sclerosis companies, including Kyowa Kirin, GSK, AstraZeneca, Amgen (Horizon Therapeutics), Mitsubishi Tanabe Pharma, Roche (Genentech), Kiniksa Pharmaceuticals, Boehringer Ingelheim, aTyr Pharma, Kyorin Pharmaceutical, Cumberland Pharmaceuticals, among others.
Systemic Sclerosis Clinical Trial Analysis
Systemic Sclerosis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key systemic sclerosis companies, including Eicos Sciences, Beijing Continent Pharmaceutical, Kyowa Kirin, Cytori therapeutics, Corbus Pharmaceuticals, Zenyaku Kogyo, Sanofi, Bayer, ASKA Pharmaceutical, Emerald Health Pharmaceuticals, United Therapeutics, Cumberland Pharmaceuticals, ILTOO Pharma, Horizon Pharmaceuticals, Janssen Biotech, Gesynta Pharma, Certa Therapeutics, Pfizer, Vicore Pharma, Seagen, CSL Behring, arGentis Pharmaceuticals, Mitsubishi Tanabe Pharma, Kadmon Pharmaceuticals, GlaxoSmithKline, Bristol-Myers Squibb, Camurus, Pfizer, Regeneron Pharmaceuticals, Regeneron Pharmaceuticals, Castle Creek Biosciences, Talaris Therapeutics, Viela Bio, Formation Biologics, Horizon Therapeutics, Chemomab Therapeutics, AnaMar, Atlantic Healthcare, D&D Pharmatech, Acceleron Pharma, Riptide Bioscience, Timber Pharmaceuticals, Tvardi Therapeutics, Accuitis Pharmaceuticals, AKL Research and Development, iBio, Blade Therapeutics, Cantargia, BriaCell Therapeutics, Leadiant Biosciences, among others.
Systemic Sclerosis-associated Interstitial Lung Disease Market
Systemic Sclerosis-associated Interstitial Lung Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key SSc-ILD companies, including Genentech Inc., Boehringer Ingelheim, Prometheus Biosciences Inc., Roche, Acceleron Pharma, Talaris Therapeutics, Kadmon Corporation, among others.
Systemic Sclerosis-associated Interstitial Lung Disease Clinical Trial Analysis
Systemic Sclerosis-associated Interstitial Lung Disease Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key SSc-ILD companies, including Genentech Inc., Boehringer Ingelheim, Prometheus Biosciences Inc., Roche, Acceleron Pharma, Talaris Therapeutics, Kadmon Corporation, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Pipeline Assessment
Healthcare Licensing Services
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter
CONTACT: Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
 Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. IndiaEveningPost.com takes no editorial responsibility for the same.
Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. IndiaEveningPost.com takes no editorial responsibility for the same.
