Comparator Drug Sourcing Market to Reach USD 2.72 Billion by 2033, Driven by Rising Global Clinical Trials and Outsourcing Trends – SNS Insider

Global Comparator Drug Sourcing Market to Grow at 8.47% CAGR as Demand for Effective, Compliant, and On-Time Clinical Trial Supply Solutions Increases.
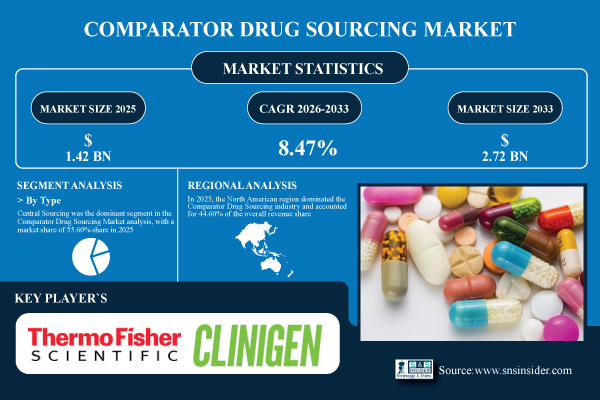
Austin, Texas, Jan. 23, 2026 (GLOBE NEWSWIRE) — As per SNS Insider, the Comparator Drug Sourcing Market was valued at USD 1.42 billion in 2025 and is projected to reach USD 2.72 billion by 2033, growing at a compound annual growth rate (CAGR) of 8.47% during the forecast period of 2026-2033. The market is growing steadily due to the increasing focus of pharmaceutical and biotech companies on global clinical trials, regulatory compliance, and effective sourcing strategies to support complex study designs.
Comparator Drug Sourcing Market Size and Forecast:
- Market Size in 2025: USD 1.42 Billion
- Market Size by 2033: USD 2.72 Billion
- CAGR: 8.47% from 2026 to 2033
- Base Year: 2025
- Forecast Period: 2026–2033
- Historical Data: 2022–2024
Request a Free Comparator Drug Sourcing Market Sample Report: https://www.snsinsider.com/sample-request/9141
The U.S. Comparator Drug Sourcing Market was valued at USD 0.53 billion in 2025 and is expected to reach USD 1.00 billion by 2033, growing at a CAGR of 8.34% from 2026 to 2033. This is aided by the high number of clinical trials, high investment in pharmaceutical R&D, and the availability of sophisticated sourcing and regulatory infrastructure that enables effective sourcing of comparators for clinical trials.
Increasing Global Clinical Trial Volume and Outsourcing Trends Drive Market Growth
The world market for sourcing comparator drugs is gaining traction owing to the rise in the number of multinational and late-stage clinical trials, the complexity of biologic and specialty drugs, and the increasing dependence on third-party partners for sourcing, packaging, and distribution. Sponsors are focusing on sourcing solutions that are reliable, compliant, and cost-effective.
Key growth drivers include:
- Increasing number of global and multi-center clinical trials
- Rising complexity of biologics, biosimilars, and specialty drugs
- Growing preference for outsourcing clinical supply chain operations
- Need for regulatory compliance and traceability across regions
- Demand for faster study start-up and on-time drug delivery
Comparator drug sourcing services remained in high demand in 2025, driven by the growing number of cancer, rare disease, and biologic clinical trials.
Segment Highlights
By Type:
Central Sourcing led the market with a 55.60% share in 2025, due to its efficiency, regulatory uniformity, and scalability in global trials.
Local Sourcing is anticipated to register the highest growth rate, with a CAGR of 8.93%, due to the rising need for regional-specific compliance, shorter delivery times, and simplified import procedures.
By Application:
Drug Producers and Manufacturers held the largest revenue share in 2025, driven by their active engagement in multinational and late-stage clinical trials and their strong control over global supply chains. Their never-ending demand for quality, compliance, and execution drives the market’s continuous growth.
Contract Manufacturing Organizations (CMOs) are expected to be the fastest-growing segment, as pharmaceutical companies increasingly turn to outsourcing clinical supply functions to cut costs and optimize operations.
Need Any Customization Research on Comparator Drug Sourcing Market, Enquire Now: https://www.snsinsider.com/enquiry/9141
North America Leads While Asia-Pacific Region Becomes Fastest-Growing Region
North America represented 44.60% of the total market in 2025, due to the large number of clinical trials, a complex regulatory system, and the presence of large pharmaceutical and biotech companies. The high investment in R&D, logistics infrastructure, and the widespread use of centralized sourcing models continue to favor the region.
The Asia-Pacific market is expected to witness the highest growth rate, with a CAGR of 9.03%, driven by the rising number of clinical trials, growing R&D investments, expanding patient base, and favorable regulatory policies in countries like China, India, South Korea, and Australia.
Major Players Analysis Listed in the Comparator Drug Sourcing Market Report are
- Thermo Fisher Scientific Inc.
- Clinigen Group plc
- Inceptua Group
- Uniphar Clinical
- Catalent, Inc.
- Clinical Services International (CSI)
- BAP Pharma Limited
- Oximio
- Bionical Emas
- Alcura
- Myonex
- Cencora, Inc.
- Sharp Services, LLC
- ADAllen Pharma
- RxSolutions Division
- PCI Pharma Services
- Quotient Sciences
- Fisher Clinical Services
- Sourcing4U
- Global Clinical Supplies Group (GCSG)
Recent Developments:
- 2024: The PPD and clinical trial supply business of Thermo Fisher Scientific made the company a leading supplier for clinical trials, thanks to its strong sourcing of comparator drugs and logistics capabilities.
- 2025: Clinigen remains at the forefront of global comparator drug sourcing, providing easy access to branded and generic comparator drugs and logistics support for clinical trial sponsors.
Purchase the Comparator Drug Sourcing Market Report Now: https://www.snsinsider.com/checkout/9141
Exclusive Sections of the Report (The USPs):
- AVERAGE SOURCING COST BENCHMARKS BY DRUG TYPE – enables you to compare sourcing costs for generic, branded, and biologic comparator drugs.
- PRICING MODEL STRUCTURE ANALYSIS – assists in analyzing unit pricing, volume discount pricing, and contract pricing models to optimize sourcing efficiency and cost management.
- SUPPLIER PRICE BENCHMARKING INSIGHTS – assists you in evaluating price differences among major global and regional suppliers, thus facilitating effective supplier selection and negotiation.
- PROCUREMENT COST FORECASTING INDICATORS – assists you in forecasting future sourcing cost trends influenced by regulatory changes, inflation, and supply chain volatility.
- PRICE VOLATILITY & RISK ASSESSMENT METRICS – assists you in determining the comparator products that are more vulnerable to pricing volatility, thus enhancing risk management and sourcing.
- COST OPTIMIZATION & STRATEGIC SOURCING INSIGHTS – assists you in optimizing the balance between cost, regulatory, and supply factors for each phase of clinical trials.
About the Report
The Comparator Drug Sourcing Market Report delivers comprehensive insights, including:
- Market size and forecasts (2022–2032)
- Detailed segmentation and regional analysis
- Competitive benchmarking and company profiling
- Industry trends, opportunities, and challenges
- Strategic insights for sponsors, CROs, and service providers
Access Complete Report Details of Comparator Drug Sourcing Market Analysis & Outlook: https://www.snsinsider.com/reports/comparator-drug-sourcing-market-9141
About Us:
SNS Insider is one of the leading market research and consulting agencies globally. Our mission is to provide clients with accurate, actionable insights to navigate changing market dynamics. We deliver high-quality market intelligence through a combination of primary research, expert interviews, surveys, and advanced analytical methodologies.
CONTACT: Contact Us: Rohan Jadhav - Principal Consultant Phone: +1-315 636 4242 (US) | +44-20 3290 5010 (UK) Email: info@snsinsider.com
 Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. IndiaEveningPost.com takes no editorial responsibility for the same.
Disclaimer: The above press release comes to you under an arrangement with GlobeNewswire. IndiaEveningPost.com takes no editorial responsibility for the same.
